By visiting our site, you agree to our privacy policy regarding cookies, tracking statistics, etc.
Thanks to Haermonics, heart surgery patients are a step closer to avoiding all too prevalent post-operative complications. Haermonics, a clinical stage medical device company focused on preventing post-operative complications after open heart surgery, and Amsterdam UMC (study sponsor) have successfully included the first patient in their FLUID multicenter clinical trial. Dr. D.R. Koolbergen, congenital cardiothoracic surgeon at Amsterdam UMC and inventor of the technology, piloted the recently developed investigational device with their first patient.
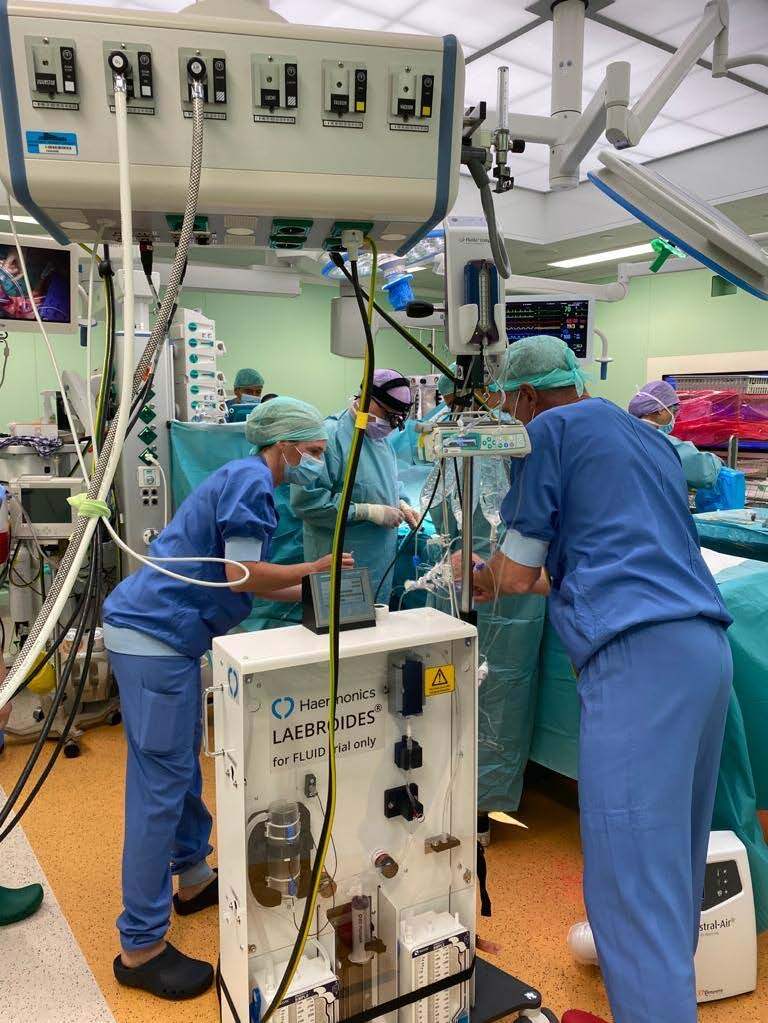
The FLUID (FLUsh with Investigational Device) trial tests out an innovative postoperative technology to improve wound drainage. Already proven in a prototype setting with over 340 patients, it is now automated to ensure scalability. The technology comprehends novel wound drainage to ensure patient safety and produces new patient monitoring data to give clinicians extra clinical information about the patient’s status.
“Blood loss is the silent cause of many complications after heart surgery,” says Wouter Markus, CEO of Haermonics, “Every year, 30,000 patients worldwide die from post-operative heart surgery complications that are preventable, costing $22B annually and untold misery. This trial brings us a big step closer to changing that story. After all, the smartest way to solve a problem is to prevent it.”
The multi-center FLUID trial aims to strengthen the evidence that this innovative wound drainage system is able to reduce excessive bleeding and other postoperative complications such as re-operations. Amsterdam UMC, LUMC and the St Antonius hospital in Nieuwegein are participating. Two earlier randomized trials have proven that the technology reduces post-operative blood loss by more than 50% and prevent occlusion of the outflow drainage tubes, which is the cause of complications in17% of all open-heart surgeries.
Haermonics’ achievement is built on more than a decade of scientific and clinical research conducted at the Amsterdam UMC hospital and Haermonics’ research facilities. Haermonics’ partner Demcon developed the investigational device, which is used during the first hours after surgery. The device controls a flushing process by which the internal wound is cleaned from blood and clots, ensuring the patency of the wound drainage system. It is the accumulation of blood and clots in the space around the heart that is responsible for many, sometimes lifethreatening complications.